Accès gratuit
| Numéro |
J. Soc. Biol.
Volume 202, Numéro 2, 2008
|
|
|---|---|---|
| Page(s) | 119 - 128 | |
| DOI | https://doi.org/10.1051/jbio:2008015 | |
| Publié en ligne | 13 juin 2008 | |
- Abdullah M., Widgren E.E. & O'Rand M.G., A mammalian sperm lectin related to rat hepatocyte lectin-2/3. Purification from rabbit testis and identification as a zona binding protein. Mol. Cell Biochem., 1991, 103, 155-161. [Google Scholar]
- Auer J., Senechal H., Desvaux F.X., Albert M. & De Almeida M., Isolation and characterisation of two sperm membrane proteins recognised by sperm-associated antibodies in infertile men. Mol. Reprod. Dev., 2000, 57, 393-405. [Google Scholar]
- Auer J., Camoin L., Courtot A.M., Hotellier F. & De Almeida M., Evidence that P36, a human sperm acrosomal antigen involved in the fertilization process is triosephosphate isomerase. Mol. Reprod. Dev., 2004, 68, 515-523. [Google Scholar]
- Avilés M., Jaber L., Castells M., Ballesta J. & Kan F.W., Modifications of carbohydrate residues of ZP2 and ZP3 glycoproteins in the mouse zona pellucida after fertilization. Biol. Reprod., 1997, 57,1155-1163. [Google Scholar]
- Baba T., Azuma S., Kashiwabara S. & Toyoda Y., Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem., 1994, 269, 31845-31849. [Google Scholar]
- Bauskin A.R., Franken D.R., Eberspaecher U. & Donner P., Characterization of human zona pellucida glycoproteins. Mol. Hum. Reprod., 1999, 5, 534-540. [Google Scholar]
- Benoff S., Carbohydrates and fertilization: an overview. Mol. Hum. Reprod., 1997, 7, 599-637. [Google Scholar]
- Benoff S., Cooper G.W., Hurley I., Napolitano B., Rosenfeld D.L., Scholl G.M. & Hershlag A., Human sperm fertilizing potential in vitro is correlated with differential expression of a head-specific mannose-ligand receptor. Fertil. Steril., 1993, 59, 854-862 [Google Scholar]
- Bleil J.D. & Wassarman P.M., Galactose at the nonreducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein's sperm receptor activity. Proc. Natl. Acad. Sci. USA, 1988, 18, 6778-6782. [CrossRef] [Google Scholar]
- Bleil J.D. & Wassarman P.M., Identification of a ZP3-binding protein on acrosome-intact mouse sperm by photoaffinity crosslinking. Proc. Natl. Acad. Sci. USA, 1990, 87, 5563-5567. [CrossRef] [Google Scholar]
- Boué F. & Sullivan R., Cases of human infertility are associated with the absence of P34H an epididymal sperm antigen. Biol. Reprod., 1996, 54, 1018-1024. [Google Scholar]
- Boué F., Bérubé B., De Lamirande E., Gagnon C. & Sullivan R., Human sperm-zona pellucida interaction is inhibited by an antiserum against a hamster sperm protein. Biol. Reprod., 1994, 51, 577-587. [Google Scholar]
- Burks D.J., Carballada R., Moore H.D. & Saling P.M., Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science, 1995, 269, 83-86 [Google Scholar]
- Chen J., Litscher E.S. & Wassarman P.M., Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc. Natl. Acad. Sci. USA, 1998, 95, 6193-6197. [CrossRef] [Google Scholar]
- Cheng A., Le T., Palcios M., Bookbinder L.H., Wassarman P.M. & Bleil J.D., Sperm-egg recognition in the mouse : characterization of sp56, a sperm protein having specific affinity for ZP3. J. Cell Biol., 1994, 125, 867-878. [Google Scholar]
- Clark G.F. & Dell A., Molecular models for murine sperm-egg binding. J. Biol. Chem., 2006, 281, 13853-13856. [Google Scholar]
- Diekman A.B, Westbrook-Case V.A., Naaby-Hansen S., Klotz K.L., Flickinger C.J. & Herr J.C., Biochemical characterization of sperm agglutination antigen-1, a human sperm surface antigen implicated in gamete interactions. Biol. Reprod., 1997, 57, 1136-1144. [Google Scholar]
- Dostálová Z., Calvete J.J., Sanz L. & Töpfer-Petersen E., Boar spermadhesin AWN-1. Oligosaccharide and zona pellucida binding characteristics. Eur. J. Biochem., 1995, 230, 329-336. [Google Scholar]
- Easton R.L., Patankar M.S., Lattanzio F.A., Leaven T.H., Morris H.R., Clark G.F. & Dell A., Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J. Biol. Chem., 2000, 275, 7731-7742 [Google Scholar]
- Ensslin M., Calvete J.J., Thole H.H., Sierralta W.D., Adermann K., Sanz L. & Topfer-Petersen E., Identification by affinity chromatography of boar sperm membrane-associated proteins bound to immobilized porcine zona pellucida. Mapping of the phosphorylethanolamine-binding region of spermadhesin AWN. Biol. Chem. Hoppe. Seyler., 1995, 376, 733-738. [Google Scholar]
- Finaz C., Martin-Ruiz C. & Lefevre A., Interaction des gamètes et protéines de reconnaissance. Médecine/Sciences, 1998, 14, 175-182. [CrossRef] [Google Scholar]
- Florman H.M. & Wassarman P.M., O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell, 1985, 41, 313-324. [CrossRef] [PubMed] [Google Scholar]
- Galindo B.E., Vacquier V.D. & Swanson W.J., Positive selection in the egg receptor for abalone sperm lysin. Proc. Natl. Acad. Sci. USA, 2003, 100, 4639-4643. [CrossRef] [Google Scholar]
- Gavrilets S., Rapid evolution of reproductive barriers driven by sexual conflict. Nature, 2000, 403, 886-889. [CrossRef] [PubMed] [Google Scholar]
- Gitlits V.M., Toh B.H., Loveland K.L. & Sentry J.W., The glycolytic enzyme enolase is present in sperm tail and displays nucleotide-dependent association with microtubules. Eur. J. Cell Biol., 2000, 79, 104-111. [Google Scholar]
- Gomendio M., Martin-Coello J., Crespo C., Magana C. & Roldan E.R.S., Sperm competition enhances functional capacity of mammalian spermatozoa. Proc. Natl. Acad. Sci. USA, 2006, 103, 15113-15117. [Google Scholar]
- Gopalakrishnan B., Aravinda S., Pawshe C.H., Totey S.M., Nagpal S., Salunke D.M. & Shaha C., Studies on glutathione S-transferases important for sperm function: evidence of catalytic activity-independent functions. Biochem. J., 1998, 329, 231-241. [Google Scholar]
- Greve J.M. & Wassarman P.M., Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J. Mol. Biol., 1985, 181, 253-264. [Google Scholar]
- Gupta S.K., Yurewicz E.C., Sacco A.G., Kaul R., Jethanandani P. & Govind C.K., Human zona pellucida glycoproteins: characterization using antibodies against recombinant non-human primate ZP1, ZP2 and ZP3. Mol. Hum. Reprod., 1998, 4, 1058-1064. [Google Scholar]
- Hall J.L., Engel D. & Naz R.K., Significance of antibodies against human sperm FA-1 antigen in immunoinfertility. Arch. Androl., 1994, 32, 25-30. [Google Scholar]
- Hardy D.M. & Garbers D.L., Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J. Biol. Chem., 1994, 269, 19000-19004. [Google Scholar]
- Harris J.D., Seid C.A., Fontenot G.K. & Liu H.F., Expression and purification of recombinant human zona pellucida proteins. Protein Expr. Purif., 1999, 16, 298-307. [Google Scholar]
- Hemachand T. & Shaha C. Functional role of sperm surface glutathione S-transferases and extracellular glutathione in the haploid spermatozoa under oxidative stress. FEBS Lett., 2003, 538, 14-18. [Google Scholar]
- Hemachand T., Gopalakrishnan B., Salunke D.M., Totey S.M. & Shaha C. Sperm plasma-membrane-associated glutathione S-transferases as gamete recognition molecules . J. Cell Sci., 2002, 115, 2053-2065. [Google Scholar]
- Hershlag A., Cooper G.W. & Benoff S., Pregnancy following discontinuation of a calcium channel blocker in the male partner. Hum. Reprod., 1995, 10, 599-606 [Google Scholar]
- Hershlag A., Scholl G.M., Jacob A., Mandel F.S., Guhring P., Paine T., Cooper GW. & Benoff S., Mannose ligand receptor assay as a test to predict fertilization in vitro: a prospective study. Fertil. Steril., 1998, 70, 482-491. [Google Scholar]
- Hoodbhoy T. & Dean J., Focus on fertilization: insights into the molecular basis of sperm-egg recognition in mammals. Reproduction, 2004, 127, 417-422 [Google Scholar]
- Hoodbhoy T., Joshi S., Boja E.S., Williams S.A., Stanley P. & Dean J., Human sperm do not bind to rat zonae pellucidae despite the presence of four homologous glycoproteins. J. Biol. Chem., 2005, 280, 12721-12731. [Google Scholar]
- Howes E., Pascall J.C., Engel W. & Jones R., Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization. J. Cell Sci., 2001, 114, 4127-4136. [Google Scholar]
- Hunnicutt G.R., Primakoff P. & Myles D.G., Sperm surface protein PH-20 is bifunctional: one activity is a hyaluronidase and a second, distinct activity is required in secondary sperm-zona binding. Biol. Reprod., 1996, 55, 80-86. [Google Scholar]
-
Johnston D.S., Wright W.W., Shaper J.H., Hokke C.H., Van Den Eijinden D.H. & Joziasse D.H., Murine sperm zona binding, a fucosyl residue is required for a high- affinity sperm-binding ligand. A second site on sperm binds a nonfucosylated,
 -galactosyl-capped oligosaccharide. J. Biol. Chem., 1998, 273, 1888-1895.
[Google Scholar]
-galactosyl-capped oligosaccharide. J. Biol. Chem., 1998, 273, 1888-1895.
[Google Scholar]
- Kim J.-W., & Dang C.V., Multifaceted roles of glycolytic enzymes. Trends in biochem.Sci., 2005, 30, 142-150. [Google Scholar]
- Lefièvre L., Conner S.J., Salpekar A., Olufowobi O., Ashton P., Pavlovic B., Lenton W., Afnan M., Brewis I.A., Monk M., Hughes D.C. & Barratt C., Four zona pellucida glycoproteins are expressed in the human. Hum. Reprod., 2004, 19, 1580-1586. [Google Scholar]
- Leyton L. & Saling P., 95 kD sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell, 1989, 57, 1123-1130. [CrossRef] [PubMed] [Google Scholar]
- Liu D.Y., Garret C. & Baker H.W., Clinical application of sperm-oocyte interaction tests in in vitro fertilization-embryo transfer and intracytoplasmic sperm injection programs. Fertil. Steril., 2004, 82, 1251-1263. [Google Scholar]
- Liu D.Y., Clarke G.N., Martic M., Garrett C. & Baker H.W., Frequency of disordered zona pellucida (ZP)-induced acrosome reaction in infertile men with normal semen analysis and normal spermatozoa-ZP binding. Hum. Reprod., 2001, 16, 1185-1190. [Google Scholar]
- Lu Q. & Shur B.D., Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development, 1997, 124, 4121-4131. [PubMed] [Google Scholar]
- Lucas H., Le Pendu J., Harb J., Moreau A., Bercegeay S &, Barrière P., Identification of spermatozoa L-selectin and two potential pellucida ligands. C. R. Acad. Sci. III., 1995, 318, 795-801. [Google Scholar]
-
Macek M.B., Lopez L.C. & Shur B.D., Aggregation of
 1, 4- galactosyltransferase on mouse sperm induces the acrosome reaction. Dev. Biol., 1991, 147, 440-444.
[Google Scholar]
1, 4- galactosyltransferase on mouse sperm induces the acrosome reaction. Dev. Biol., 1991, 147, 440-444.
[Google Scholar]
- Makalowski W. & Boguski M.S., Evolutionary parameters of the transcribed mammalian genome: an analysis of 2,820 orthologous rodent and human sequences. Proc. Natl. Acad. Sci. USA, 1998, 95, 9407-9412. [CrossRef] [Google Scholar]
- Martin Ruiz C., Duquenne C., Treton D., Lefèvre A. & Finaz C., SOB3, a human sperm protein involved in zona pellucida binding: physiological and biochemical analysis, purification. Mol. Reprod. Dev., 1998, 49, 286-297. [Google Scholar]
- Menge A.C., Christman G.M., Ohl D.A. & Naz R.K., Fertilization antigen-1 removes antisperm autoantibodies from spermatozoa of infertile men and results in increased rates of acrosome reaction. Fertil. Steril., 1999, 71, 256-260. [Google Scholar]
-
Miller D.J., Macek M.B. & Shur B.D., Complementarity between sperm surface
 1, 4- galactosyl-transferase and egg coat ZP3 mediates sperm-egg binding. Nature, 1992, 357, 589-593.
[CrossRef]
[PubMed]
[Google Scholar]
1, 4- galactosyl-transferase and egg coat ZP3 mediates sperm-egg binding. Nature, 1992, 357, 589-593.
[CrossRef]
[PubMed]
[Google Scholar]
-
Miller D.J., Gong X.H., Decker G. & Shur B.D., Egg cortical granule
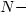 acetylglucosaminidase is required for the mouse zona block to polyspermy. J. Cell Biol., 1993, 123, 1431-1440.
[CrossRef]
[PubMed]
[Google Scholar]
acetylglucosaminidase is required for the mouse zona block to polyspermy. J. Cell Biol., 1993, 123, 1431-1440.
[CrossRef]
[PubMed]
[Google Scholar]
- Mori E., Kashiwabara S., Baba T., Inagaki Y. & Mori T., Amino acid sequences of porcine Sp38 and proacrosin required for binding to the zona pellucida. Dev. Biol., 1995, 168, 575-583. [Google Scholar]
- Mortillo S. & Wassarman P.M., Differential binding of gold-labeled zona pellucida glycoproteins mZP2 and mZP3 to mouse sperm membrane compartments. Development, 1991, 113, 141-149. [Google Scholar]
- Nakano M., Yonezawa N., Hatanaka Y. & Noguchi S., Structure and function of the N-linked carbohydrate chains of pig zona pellucida glycoproteins. J. Reprod. Fertil. Suppl., 1996, 50, 25-34. [PubMed] [Google Scholar]
- Naz R.K. & Ahmad K., Molecular identities of human sperm proteins that bind human zona pellucida: nature of sperm-zona interaction, tyrosine kinase activity, and involvement of FA-1. Mol. Reprod. Dev., 1994, 39, 397-408. [Google Scholar]
- Nixon B., Asquith K.L. & Aitken R.J., The role of molecular chaperones in mouse sperm–egg interactions. Mol. Cell. Endocrinol., 2005, 240, 1-10. [CrossRef] [PubMed] [Google Scholar]
- Noguchi S., Hatanaka Y., Tobita T. & Nakano M., Structural analysis of the N-linked carbohydrate chains of the 55 kDa glycoprotein family (PZP3) from porcine zona pellucida. Eur. J. Biochem., 1992, 204, 1089-1100. [Google Scholar]
- O'Rand M.G., Matthews J.E., Welch J.E. & Fisher S.J., Identification of zona binding proteins of rabbit, pig, human, and mouse spermatozoa on nitrocellulose blots. J. Exp. Zool., 1985, 235, 423-428. [Google Scholar]
- Patrat C., Serres C. & Jouannet P., The acrosome reaction in human spermatozoa. Biol. Cell., 2000, 92, 255-266. [Google Scholar]
- Peterson R.N., Campbell P., Hunt W.P. & Bozzola J.J., Two-dimensional polyacrylamide gel electrophoresis characterization of APz, a sperm protein involved in zona binding in the pig and evidence for its binding to specific zona glycoproteins. Mol. Reprod. Dev., 1991, 28, 260-71. [Google Scholar]
- Rao A.V. & Shaha C., Multiple glutathione S-transferase isoforms are present on male germ cell plasma membrane. FEBS Lett., 2001, 507, 174-180. [Google Scholar]
- Rattanachaiyanont M., Weerachatyanukul W., Léveillé M.C., Taylor T., D'Amours D., Rivers D., Leader A. & Tanphaichitr N., Anti-SLIP1-reactive proteins exist on human spermatozoa and are involved in zona pellucida binding. Mol. Hum. Reprod., 2001, 7, 633-640. [Google Scholar]
- Richardson R.T., Yamasaki N. & O'Rand M.G., Sequence of a rabbit sperm zona pellucida binding protein and localization during the acrosome reaction. Dev. Biol., 1994, 165, 688-701. [Google Scholar]
- Shabanowitz R.B. & O'Rand M.G., Molecular changes in the human zona pellucida associated with fertilization and human sperm-zona interactions. Ann. N Y Acad. Sci., 1988, 541, 621-632. [Google Scholar]
- Sinowatz F., Wessa E., Neumüller C. & Palma G., On the species specificity of sperm binding and sperm penetration of the zona pellucida. Reprod. Domest. Anim., 2003, 38, 141-146. [Google Scholar]
- Spargo S.C. & Hope R.M., Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod., 2003, 68, 358-362. [Google Scholar]
- Stein K.K., Go J.C., Lane W.S., Primakoff P. & Myles D.G., Proteomic analysis of sperm regions that mediate sperm-egg interactions. Proteomics, 2006, 6, 3533-3543. [CrossRef] [PubMed] [Google Scholar]
- Sullivan R., Legare C., Villeneuve M., Foliguet B. & Bissonnette F., Levels of P34H, a sperm protein of epididymal origin, as a predictor of conventional in vitro fertilization outcome. Fertil. Steril., 2006, 85, 1557- 1559. [Google Scholar]
- Swanson W.J. & Vacquier V.D., The rapid evolution of reproductive proteins. Nature Reviews Genetics, 2002, 3, 137-144. [Google Scholar]
- Swanson W.J., Nielsen R. & Yang Q., Pervasive adaptative evolution in mammalian fertilization proteins. Mol. Biol. Evol., 2003, 20, 18-20. [Google Scholar]
- Swanson W.J., Yang Z., Wolfner M.F. & Aquadro C.F., Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc. Natl. Acad. Sci. USA, 2001, 98, 2509-2514. [Google Scholar]
- Talbot P., Shur B.D. & Myles D.G., Cell adhesion and fertilization: steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion. Biol. Reprod., 2003, 68, 1-9. [Google Scholar]
- Tanii I., Oh-oka T., Yoshinaga K. & Toshimori K. A mouse acrosomal cortical matrix protein, MC41, has ZP2-binding activity and forms a complex with a 75-kDa serine protease. Dev. Biol., 2001, 238, 332-341. [CrossRef] [PubMed] [Google Scholar]
- Tulsiani D.R., Skudlarek M.D. & Orgebin-Crist M.C., Human sperm plasma membranes possess alpha-D-mannosidase activity but no galactosyltransferase activity. Biol. Reprod., 1990, 42, 843-858. [CrossRef] [PubMed] [Google Scholar]
- Vieira A. & Miller D.J., Gamete interaction: is it species-specific? Mol. Reprod. Dev., 2006, 73, 1422-1429. [Google Scholar]
- Wassarman P.M., Mammalian fertilization: molecular aspects of gamete adhesion, exocytosis, and fusion. Cell, 1999, 96, 175-183. [CrossRef] [PubMed] [Google Scholar]
- Wassarman P.M., Sperm receptors and fertilization in mammals. Mt Sinai J. Med., 2002, 69, 148-155. [PubMed] [Google Scholar]
- Wassarman P.M. & Litscher E.S., Sperm–egg recognition mechanisms in mammals. Curr. Top. Dev. Biol., 1995, 30, 1-19. [Google Scholar]
- Wyckoff G.J., Wang W. & Wu C-I., Rapid evolution of male reproductive genes in the descent of man. Nature, 2000, 403, 304-308. [CrossRef] [PubMed] [Google Scholar]
- Wright R.M., John E., Klotz K., Flickinger C.J. & Herr J.C., Cloning and sequencing of cDNAs coding for the human intra-acrosomal antigen SP-10. Biol. Reprod., 1990, 42, 693-701. [CrossRef] [PubMed] [Google Scholar]
- Yanagimachi R., Mammalian fertilization. In Knobil E. & Neill J.D. (eds) The Physiology of Reproduction. 1994, 2nd edn., Raven Press Ltd, New York, 189-317. [Google Scholar]
- Yonezawa N., Kanai S. & Nakano M., Structural significance of N-glycans of the zona species-selective recognition spermatozoa between pig and cattle. Soc. Reprod. Fertil. Suppl., 2007, 63, 217-228. [Google Scholar]
- Yurewicz E.C., Sacco A.G., Gupta S.K., Xu N. & Gage D.A., Hetero-oligomerization-dependent binding of pig oocyte zona pellucida glycoproteins ZPB and ZPC to boar sperm membrane vesicles. J. Biol. Chem., 1998, 273, 7488-7494. [CrossRef] [PubMed] [Google Scholar]
- Zhu X. & Naz R.K., Fertilization antigen-1: c DNA cloning, testis-specific expression, and immunocontraceptive effects. Proc. Natl. Acad. Sci. USA, 1997, 94, 4704-4709. [CrossRef] [Google Scholar]
- Zouari R. & De Almeida M., Effect of sperm-associated antibodies on human sperm ability to bind to zona pellucida and to penetrate zona-free hamster oocytes. J. Reprod. Immunol., 1993, 24, 175-186. [Google Scholar]
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.


