Accès gratuit
| Numéro |
Biologie Aujourd'hui
Volume 206, Numéro 4, 2012
Journée Claude Bernard 2011
|
|
|---|---|---|
| Page(s) | 323 - 335 | |
| DOI | https://doi.org/10.1051/jbio/2012031 | |
| Publié en ligne | 19 février 2013 | |
- Agrell D., Larsson C., Larsson M., Mackown C., Rufty T., Initial kinetics of N-15-Nitrate labeling of root and shoot N fractions of barley cultured at different relative addition rates of nitrate-N. Plant Physiol Biochem, 1997, 35, 923–932. [Google Scholar]
-
Albrecht V., Ritz O., Linder S., Harter K., Kudla J., The NAF domain defines a novel protein-protein interaction module conserved in
 -regulated kinases. EMBO J, 2001, 20, 1051–1063.
[CrossRef]
[PubMed]
[Google Scholar]
-regulated kinases. EMBO J, 2001, 20, 1051–1063.
[CrossRef]
[PubMed]
[Google Scholar]
- Bernard S.M., Habash D.Z., The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol, 2009, 182, 608–620. [CrossRef] [PubMed] [Google Scholar]
- Bloom A.J., Nitrogen as a limiting factor: Crop acquisition of ammonium and nitrate, in Ecology in Agriculture. In L.E. Jackson (Ed.), Agricultural Ecology, 1997, Academic Press, San Diego, pp. 145–172. [Google Scholar]
- Bothe H., Metabolism in inorganic nitrogen compounds. Progr Botany, 1987, 52, 122–137. [Google Scholar]
- Brenner W.G., Romanov G.A., Köllmer I., Bürkle L., Schmülling T., Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J, 2005, 44, 314–33. [CrossRef] [PubMed] [Google Scholar]
- Camargo A., Llamas A., Schnell R.A., Higuera J.J., González-Ballester, D., Lefebvre P.A., Fernandez E., Galvan A., Nitrate signaling by the regulatory gene NIT2 in Chlamydomonas. Plant Cell, 2007, 19, 3491–3503. [CrossRef] [PubMed] [Google Scholar]
- Casimiro I., Marchant A., Bhalerao R.P., Beeckman T., Dhooge S., Swarup R., Graham N., Inzé D., Sandberg G., Casero P.J., Bennett M., Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell, 2001, 13, 843–852. [CrossRef] [PubMed] [Google Scholar]
- Castaings L., Camargo A., Pocholle D., Gaudon V., Texier Y., Boutet-Mercey S., Taconnat L., Renou J.P., Daniel-Vedele F., Fernandez E., Meyer C., Krapp A., The nodule inception- like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J, 2009, 57, 426–435. [CrossRef] [PubMed] [Google Scholar]
-
Cerezo M., Tillard P., Filleur S., Muños S., Daniel-Vedele F., Gojon A., Major alterations of the regulation of root
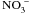 uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol, 2001, 127, 262–271.
[CrossRef]
[PubMed]
[Google Scholar]
uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol, 2001, 127, 262–271.
[CrossRef]
[PubMed]
[Google Scholar]
- Chia D.W., Yoder T.J., Reiter W.D., Gibson S.I., Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta, 2000, 211, 743–751. [CrossRef] [PubMed] [Google Scholar]
- Chopin F., Wirth J., Dorbe M., Lejay L., Krapp A., Gojon, A., Daniel-Vedele, F., The Arabidopsis nitrate transporter AtNRT2.1 is targeted to the root plasma membrane. Plant Physiol Biochem, 2007, 45, 630–635. [CrossRef] [PubMed] [Google Scholar]
- Clarkson D.T., Regulation of the absorption and release of nitrate by plant cells: a review of current ideas and methodology. In H. Lambers, J. Neeteson, I. Stulens (Eds.), Fundamental, Ecological and Agricultural Aspects of Nitrogen Metabolism in Higher Plants, 1986, F. Martinus Nijhoof Publishers, Dordrech, pp. 2–27. [Google Scholar]
- Cookson S.J., Williams L.E., Miller A.J., Light-dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiol, 2005, 138, 1097–1105. [CrossRef] [PubMed] [Google Scholar]
- Coruzzi G.M., Bush D., Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol, 2001, 125, 61–46. [CrossRef] [PubMed] [Google Scholar]
- Coruzzi G.M., Zhou L., Carbon and nitrogen sensing and signaling in plants: emerging « matrix effects » . Curr Opinion Plant Biol, 2001, 4, 247–253. [CrossRef] [Google Scholar]
- Crawford N.M., Forde B.G., Molecular and developmental Biology of Inorganic Nitrogen. In C.R. Somerville, E.M Meyerowitz (Eds.), The Arabidopsis Book, 2002, Rockville M.D. American Society of Plant Biologists, pp. 1–25. [Google Scholar]
- De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H., The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature, 2006, 442, 939–942. [CrossRef] [PubMed] [Google Scholar]
- Diaz C., Saliba-Colombani V., Loudet O., Belluomo P., Moreau L., Daniel-Vedele F., Morot-Gaudry J.F., Masclaux-Daubresse C., Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana. Plant Cell Physiol, 2006, 47, 74–83. [Google Scholar]
- Dubois F., Tercé-Laforgue T., Gonzalez-Moro M.B., Estavillo M.B., Sangwan R., Gallais A., Hirel B., Glutamate dehydrogenase in plants; is there a new story for an old enzyme? Plant Physiol Biochem, 2003, 41, 565–576. [CrossRef] [Google Scholar]
- Drew M.C., Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol, 1975, 75, 479–490. [CrossRef] [Google Scholar]
- Filleur S., Dorbe M.F., Cerezo M., Orsel M., Granier F., Gojon A., Daniel-Vedele F., An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Letters, 2001, 489, 220–224. [CrossRef] [PubMed] [Google Scholar]
- Fontaine J.X., Saladino F., Agrimonti C., Bedu M., Tercé-Laforgue T., Tétu T., Hirel B., Restivo F.M., Dubois F., Control of the synthesis and subcellular targeting of the two GDH genes products in leaves and stems of Nicotiana plumbaginifolia and Arabidopsis thaliana. Plant Cell Physiol, 2006, 47, 410–418. [CrossRef] [PubMed] [Google Scholar]
- Forde B.G., The role of long-distance signalling in plant responses to nitrate and other nutrients. J Exp Bot, 2002, 53, 39–43. [CrossRef] [PubMed] [Google Scholar]
- Foyer C.H., Parry M., Markers and signals associated with nitrogen assimilation in higher plants. J Exp Bot, 2003, 54, 585–593. [CrossRef] [PubMed] [Google Scholar]
- Geelen D., Lurin C., Bouchez D., Frachisse J.M., Lelièvre F., Courtial B., Barbier-Brygoo H., Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J, 2000, 21, 259–267. [CrossRef] [PubMed] [Google Scholar]
- Gifford M.L., Dean A., Gutierrez R.A., Coruzzi G.M., Birnbaum K.D., Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA, 2008, 105, 803–808. [CrossRef] [Google Scholar]
- Glass A.D.M., Shaff J.E., Kochian, L.V., Studies of the Uptake of Nitrate in Barley: IV. Electrophysiology. Plant Physiol, 1992, 99, 456–463. [CrossRef] [PubMed] [Google Scholar]
- Glynn C., Herms D.A., Orians C.M., Hansen R.C., Larsson S., Testing the growth-differentiation balance hypothesis: dynamic responses of willows to nutrient availability. New Phytol, 2007, 176, 623–634. [CrossRef] [PubMed] [Google Scholar]
- Herdel K., Schmidt P., Feil R., Mohr A., Schurr U., Dynamics of concentrations and nutrient fluxes in the xylem of Ricinus communis-diurnal course, impact of nutrient availability and nutrient uptake. Plant Cell Environ, 2001, 24, 41–52. [CrossRef] [Google Scholar]
- Herrmann K.M., Weaver L.M., The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol, 1999, 50, 473–503. [CrossRef] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F., CHL1 functions as a nitrate sensor in plants. Cell, 2009, 138, 1184–1194. [CrossRef] [MathSciNet] [PubMed] [Google Scholar]
- Hoai N.T.T, Shim I.S., Kobayashi K., Usui K., Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa) seedlings. Plant Growth Regul, 2003, 41, 159–164. [CrossRef] [Google Scholar]
- Hsieh M.H., Lam H.M., van de Loo F.J., Coruzzi G., A PII-like protein in Arabidopsis: Putative role in nitrogen sensing. Proc Natl Acad Sci USA, 1998, 95, 13965–13970. [CrossRef] [Google Scholar]
- Hu H.C., Wang Y.Y., Tsay Y.F., AtCIPK8, a CBL-interating protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J, 2009, 57, 264–278. [CrossRef] [PubMed] [Google Scholar]
- Huang N.C., Liu K.H., Lo H.J., Tsay, Y.F., Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell, 1999, 11, 1381–1392. [PubMed] [Google Scholar]
- Huppe H.C., Farr T.J., Turpin D.H., Coordination of chloroplastic metabolism in N-limited Chlamydomonas reinhardtii by redox modulation. 2. Redox modulation activates the oxidative pentose phosphate pathway during photosynthetic nitrate assimilation. Plant Physiol, 1994, 105, 1043–1048. [PubMed] [Google Scholar]
- IPCC, Intergovernmental Panel on Climate Change Report 2007. http://www.ipcc.ch/publications˙and˙ data/publications˙and˙data˙ reports.shtml [Google Scholar]
- Ikram S., Bedu M., Daniel-Vedele F., Chaillou S., Chardon F., Natural variation of Arabidopsis response to nitrogen availability. J Exp Bot, 2012, 63, 91–105. [CrossRef] [PubMed] [Google Scholar]
- Johnson P.T., Chase J.M., Dosch K.L., Hartson R.B., Gross J.A., Larson D.J., Sutherland D.R., Carpenter S.R., Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA, 2007, 104, 15781–15786. [CrossRef] [Google Scholar]
- Kant S., Peng M., Rothstein S.J., Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in Arabidopsis. PLoS Genetics, 2011, 7, e1002021. [CrossRef] [PubMed] [Google Scholar]
- Kiba T., Feria-Bourrellier A.B., Lafouge F., Lezhneva L., Boutet-Mercey S., Orsel M., Bréhaut V., Miller A., Daniel-Vedele F., Sakakibara H., Krapp A., The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell, 2012, 24, 245–258. [CrossRef] [PubMed] [Google Scholar]
- Kleinhofs A., Warner R.L., Advances in nitrate assimilation. In B. Mifflin, P. Lea (Eds.), The biochemistry of plants, 1990, Academic press, 16, pp. 89–120. [Google Scholar]
- Köhler B., Wegner L.H., Osipov V., Raschke K., Loading of nitrate into the xylem: apoplastic nitrate controls the voltage dependence of X-QUAC, the main anion conductance in xylem-parenchyma cells of barley roots. Plant J, 2002, 30, 133–142. [CrossRef] [PubMed] [Google Scholar]
- Koprivova A., Suter M., den Camp R.O., Brunold C., Kopriva S., Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiol, 2000, 122, 737–746. [CrossRef] [PubMed] [Google Scholar]
- Krapp A., Truong H.N., Regulation of C/N interaction in model plant species. J Crop Improv, 2006, 15, 127–173. [CrossRef] [Google Scholar]
- Krapp A., Berthomé R., Orsel M., Mercey-Boutet S., Yu A., Castaings L., Elftieh S., Major H., Renou J.-P., Daniel-Vedele F., Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol, 157, 2011, 1255–1282. [CrossRef] [PubMed] [Google Scholar]
- Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., Hoyerova K., Tillard P., Leon S., Ljung K., Zazimalova E., Benkova E., Nacry P., Gojon A., Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Develop Cell, 2010a, 18, 927–937. [CrossRef] [PubMed] [Google Scholar]
- Krouk G., Mirowski P., LeCun Y., Shasha D.E., Coruzzi G.M., Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Genome Biol, 2010b, 11, R123. [CrossRef] [PubMed] [Google Scholar]
- Lawlor D.W., Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J Exp Bot, 2002, 53, 773–787. [CrossRef] [Google Scholar]
- Lawlor D.W., Konturri M., Young A.T., Photosynthesis by flag leaves of wheat in relation to protein, ribulose bisphosphate carboxylase activity and nitrogen supply. J Exp Bot, 1989, 40, 43–52. [CrossRef] [Google Scholar]
- Lea U.S., Slimestad R., Smedvig P., Lillo C., Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta, 2007, 225, 1245–1253. [CrossRef] [PubMed] [Google Scholar]
- Little D.Y., Rao H., Oliva S., Daniel-Vedele F., Krapp A., Malamy J.E., The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA, 2005, 102, 13693–13698. [CrossRef] [Google Scholar]
- Liu K.H., Huang C.Y., Tsay Y.F., CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate. Plant Cell, 1999, 11, 865–874. [PubMed] [Google Scholar]
- Liu K.H., Tsay Y.F., Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J, 2003, 22, 1005–1013. [CrossRef] [PubMed] [Google Scholar]
- Loomis W.E., Growth-differentiation balance vs. carbohydrate-nitrogen ratio. Am Soc Hortic Sci Proc, 1932, 29, 240–245. [Google Scholar]
- Loudet O., Chaillou S., Merigout P., Talbotec J., Daniel-Vedele F., Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol, 2003, 131, 345–358. [CrossRef] [PubMed] [Google Scholar]
- Loulakakis K.A., Primikirios N.I., Nikolantonakis M.A., Roubelakis-Angelakis K.A., Immunocharacterization of Vitis vinifera L. Ferredoxin-dependent glutamate synthase and its spatial and temporal changes during leaf development. Planta, 2002, 215, 630–638. [CrossRef] [PubMed] [Google Scholar]
- Lutts S., Majerus V., Kinet J.M., NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant, 1999, 105, 450–458. [Google Scholar]
- Martin T., Oswald O., Graham I.A., Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol, 2002, 128, 472–481. [CrossRef] [PubMed] [Google Scholar]
- Masclaux C., Valadier M.H., Brugière N., Morot-Gaudry J.F., Hirel B., Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta, 2000, 211, 510–518. [CrossRef] [PubMed] [Google Scholar]
- Masclaux-Daubresse C., Reisdorf-Cren M., Pageau K., Lelandais M., Grandjean O., Kronenberger J., Valadier M.-H., Feraud M., Jouglet T., Suzuki A., Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol, 2006, 140, 444–456. [CrossRef] [PubMed] [Google Scholar]
- Miflin B.J., Habash D.Z., The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot, 2002, 53, 979–987. [CrossRef] [PubMed] [Google Scholar]
- Matros A., Amme S., Kettig B., Buck-Sorlin G.H., Sonnewald U., Mock H.P., Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ, 2006, 29, 126–137. [CrossRef] [PubMed] [Google Scholar]
- Muños S., Cazettes C., Fizames C., Gaymard F., Tillard P., Lepetit M., Gojon A., Transcript profiling in the chl1–5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. Plant Cell, 2004, 16, 2433–2447. [CrossRef] [PubMed] [Google Scholar]
- Nishizawa A., Yabuta Y., Shigeoka S., Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol, 2008, 147, 1251–1263. [CrossRef] [PubMed] [Google Scholar]
- Okamoto M., Vidmar J.J., Glass A.D.M., Regulation of NRT1 gene families of Arabidopsis thaliana: responses to nitrate. Plant Cell Physiol, 2003, 44, 304–317. [CrossRef] [PubMed] [Google Scholar]
- Okamoto M., Kumar A., Li W., Wang Y., Siddiqi M.Y., Crawford N.M., Glass A.D., High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol, 2006, 140, 1036–1046. [CrossRef] [PubMed] [Google Scholar]
- Orsel M., Filleur S., Fraisier V., Daniel-Vedele F., Nitrate transport in plants: which gene and which control? J Exp Bot, 2002, 53, 825–33. [CrossRef] [PubMed] [Google Scholar]
- Orsel M., Eulenburg K., Krapp A., Daniel-Vedele F., Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta, 2004, 219, 714–721. [CrossRef] [PubMed] [Google Scholar]
- Orsel M., Chopin F., Leleu O., Smith S., Krapp A., Daniel-Vedele F., Miller A., Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein-protein interaction. Plant Physiol, 2006, 142, 1304–1317. [CrossRef] [PubMed] [Google Scholar]
- Peng M., Hannam C., Gu H., Bi Y., Rothstein, S.J., A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J, 2007, 50, 320–337. [CrossRef] [PubMed] [Google Scholar]
- Peoples M.B., Freney J.R., Mosier, A.R., Minimizing gaseous losses of nitrogen. In P.E. Bacon (Ed.), Nitrogen fertilization and the environment, 1995, Marcel Dekker Inc., New York, pp. 565–602. [Google Scholar]
- Pracharoenwattana I., Zhou W., Keech O., Francisco P.B., Udomchalothorn T., Tschoep H., Stitt M., Gibon Y., Smith S.M., Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J, 2010, 62, 785–795. [CrossRef] [PubMed] [Google Scholar]
- Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Gojon A., The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA, 2006, 103, 19206–19211. [CrossRef] [Google Scholar]
- Rouached H., Secco D., Arpat B., Poirier Y., The transcription factor PHR1 plays a key role in the regulation of sulfate shoot-to-root flux upon phosphate starvation in Arabidopsis. BMC Plant Biol, 2011, 11, 19. [CrossRef] [PubMed] [Google Scholar]
- Rouzé P., Caboche M., Nitrate reductase in higher plants: molecular approaches to function and regulations. In J. Wray (Ed.), Inducible Plant Proteins, 1992, Cambridge University Press, pp. 45–77. [Google Scholar]
- Rubin G., Tohge T., Matsuda F., Saito K., Scheible W.-R., Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell, 2009, 21, 3567–3584. [CrossRef] [PubMed] [Google Scholar]
- Sakakibara H., Takei K., Hirose N., Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci, 2006, 11, 440–448. [CrossRef] [PubMed] [Google Scholar]
- Schauser L., Roussis A., Stiller J., Stougaard J., A plant regulator controlling development of symbiotic root nodules. Nature, 1999, 401, 191–195. [Google Scholar]
- Scheible W., Lauerer M., Schulze E., Caboche M., Stitt M., Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J, 1997, 11, 671–691. [CrossRef] [Google Scholar]
- Scheible W., Morcuende R., Czechowski T., Fritz C., Osuna D., Palacios-Rojas N., Stitt M., Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol, 2004, 136, 2483–2499. [CrossRef] [PubMed] [Google Scholar]
- Segonzac C., Boyer J., Ipotesi E., Szponarski W., Tillard P., Touraine B., Sommerer N., Rossignol M., Gibrat R., Nitrate efflux at the root plasma membrane: identification of an Arabidopsis excretion transporter. Plant Cell, 2007, 19, 3760–3777. [CrossRef] [PubMed] [Google Scholar]
- Shin R., Berg R.H., Schachtman D.P., Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol, 2005, 46, 1350–1357. [CrossRef] [PubMed] [Google Scholar]
- Signora L., De Smet I., Foyer C.H., Zhang H., ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. Plant J, 2001, 28, 655–662. [CrossRef] [PubMed] [Google Scholar]
- Skopelitis D.S., Paranychianakis N.V., Paschalidis K.A., Pliakonis E.D., Delis I.D., Yakoumakis D.I., Kouvarakis A., Papadakis A.K., Stephanou E.G., Roubelakis-Angelakis K.A., Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell, 2006, 18, 2767–2781. [CrossRef] [PubMed] [Google Scholar]
- Sugiura M., Georgescu M.N., Takahashi M., A nitrite transporter associated with nitrite uptake by higher plant chloroplasts. Plant Cell Physiol, 2007, 48, 1022–1035. [CrossRef] [PubMed] [Google Scholar]
- Sulpice R., Pyl E.-T., Ishihara H., Trenkamp S., Steinfath M., Witucka-Wall H., Gibon Y., Usadel B., Poree F., Piques M.C., Von Korff M., Steinhauser M.C., Keurentjes J.J., Guenther M., Hoehne M., Selbig J., Fernie A.R., Altmann T., Stitt M., Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA, 2009, 106, 10348–10353. [CrossRef] [Google Scholar]
- Taji T., Ohsumi C., Iuchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K., Shinozaki K., Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J, 2002, 29, 417–426. [CrossRef] [PubMed] [Google Scholar]
- Takei K., Sakakibara H., Taniguchi M., Sugiyama T., Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol, 2001, 42, 85–93. [CrossRef] [PubMed] [Google Scholar]
- Takei K., Takahashi T., Sugiyama T., Yamaya T., Sakakibara H., Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin. J Exp Bot, 2002, 53, 971–977. [CrossRef] [PubMed] [Google Scholar]
- Takei K., Ueda N., Aoki K., Kuromori T., Hirayama T., Shinozaki K., Yamaya T., Sakakibara H., AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol, 2004, 45, 1053–1062. [CrossRef] [PubMed] [Google Scholar]
- Taniguchi M., Kiba T., Sakakibara H., Ueguchi C., Mizuno T., Sugiyama T., Expression of Arabidopsis response regulator homologs is induced by cytokinins and nitrate. FEBS Lett, 1998, 429, 259–262. [CrossRef] [PubMed] [Google Scholar]
- Tercé-Laforgue T., Dubois F., Ferrario-Méry S., Pou de Crecenzo M.-A., Sangwan R., Hirel B., Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration. Plant Physiol, 2004, 136, 4308–4317. [CrossRef] [PubMed] [Google Scholar]
- Tilman D., Reich P.B., Knops J., Wedin D., Mielke T., Lehman C., Diversity and productivity in a long-term grassland experiment. Science, 2001, 294, 843–845. [CrossRef] [PubMed] [Google Scholar]
- Tsay Y.F., Schroeder J.I., Feldmann K.A., Crawford, N.M., The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell, 1993, 72, 705–713. [CrossRef] [PubMed] [Google Scholar]
- Tschoep H., Gibon Y., Carillo P., Armengaud P., Szecowka M., Nunes-Nesi A., Fernie A.R., Koehl K., Stitt M., Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ, 2009, 32, 300–318. [CrossRef] [PubMed] [Google Scholar]
- Turpin D.H., Weger H.G., Huppe H.C., Interactions between photosynthesis, respiration and nitrogen assimilation. In D.T. Dennis, D.H. Turpin, D.B. Layzell (Eds.), Plant Metabolism, 1997, Longman, Singapore. [Google Scholar]
- Van der Leij M., Smith S., Miller A., Simultaneous influx of ammonium and potassium into maize roots: kinetics and interactions. Planta, 1998, 205, 64–72. [CrossRef] [Google Scholar]
- Vidal E.A., Araus V., Lu C., Parry G., Green P.J., Coruzzi G.M., Gutiérrez R.A., Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA, 2010, 107, 4477–4482. [CrossRef] [Google Scholar]
-
Von der Fecht-Bartenbach J., Bogner M., Dynowski M., Ludewig U., CLC-b-mediated
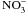 /H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol, 2010, 51, 960–968.
[CrossRef]
[PubMed]
[Google Scholar]
/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol, 2010, 51, 960–968.
[CrossRef]
[PubMed]
[Google Scholar]
- Walch-Liu P., Neumann G., Bangerth F., Engels C., Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J Exp Bot, 2000, 51, 227–237. [CrossRef] [PubMed] [Google Scholar]
- Walch-Liu P., Ivanov I.I., Filleur S., Gan Y., Remans T., Forde, B.G., Nitrogen regulation of root branching. Ann Bot, 2006, 97, 875–881. [CrossRef] [PubMed] [Google Scholar]
- Wang R., Liu D., Crawford N.M., The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA, 1998, 95, 15134–1539. [CrossRef] [Google Scholar]
- Wang R., Tischner R., Gutiérrez R.A., Hoffman M., Xing X., Chen M., Crawford N.M., Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol, 2004, 136, 2512–2522. [CrossRef] [PubMed] [Google Scholar]
- Wang R., Xing X., Wang Y., Tran A., Crawford N.M., A Genetic Screen for Nitrate-Regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol, 2009, 151, 472–478. [CrossRef] [PubMed] [Google Scholar]
- Wang Y.Y., Hsu P.K., Tsay Y.F., Uptake, allocation and signaling of nitrate. Trends Plant Sci, 2012, 17, 458–467. [CrossRef] [PubMed] [Google Scholar]
-
Widiez T., El Kafafi el S., Girin T., Berr A., Ruffel S., Krouk G., Vayssières A., Shen W.H., Coruzzi G.M., Gojon A., Lepetit M., High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root
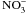 uptake is associated with changes in histone methylation. Proc Natl Acad Sci USA, 2011, 108, 13329–13334.
[CrossRef]
[Google Scholar]
uptake is associated with changes in histone methylation. Proc Natl Acad Sci USA, 2011, 108, 13329–13334.
[CrossRef]
[Google Scholar]
- Yanagisawa S., Akiyama A., Kisaka H., Uchimiya H., Miwa T., Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA, 2004, 101, 7833–7838. [CrossRef] [Google Scholar]
- Yong Z., Kotur Z., Glass A.D., Characterization of an intact two-component high-affinity nitrate transporter from Arabidopsis roots. Plant J, 2010, 63, 739–748. [CrossRef] [PubMed] [Google Scholar]
- Zhang H., Forde B.G., An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science, 1998, 279, 407–409. [CrossRef] [PubMed] [Google Scholar]
- Zhang H., Jennings A., Barlow P.W., Forde, B.G., Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA, 1999, 96, 6529–6534. [CrossRef] [Google Scholar]
- Zhang H., Rong H., Pilbeam D., Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J Exp Bot, 2007, 58, 2329–2338. [CrossRef] [PubMed] [Google Scholar]
- Zuther E., Büchel K., Hundertmark M., Stitt M., Hincha D.K., Heyer A.G., The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett, 2004, 576, 169–173. [CrossRef] [PubMed] [Google Scholar]
Les statistiques affichées correspondent au cumul d'une part des vues des résumés de l'article et d'autre part des vues et téléchargements de l'article plein-texte (PDF, Full-HTML, ePub... selon les formats disponibles) sur la platefome Vision4Press.
Les statistiques sont disponibles avec un délai de 48 à 96 heures et sont mises à jour quotidiennement en semaine.
Le chargement des statistiques peut être long.


